Periodic Table: First 20 Elements, Properties, and Classification
Lesson Overview
The periodic table is a scientific chart that arranges all chemical elements according to their atomic number and properties. Learning about their properties and classification reveals important patterns, such as reactivity, bonding behavior, and group trends. Understanding these basics builds a strong base for exploring more advanced concepts in chemistry and science.
What Is the Periodic Table?
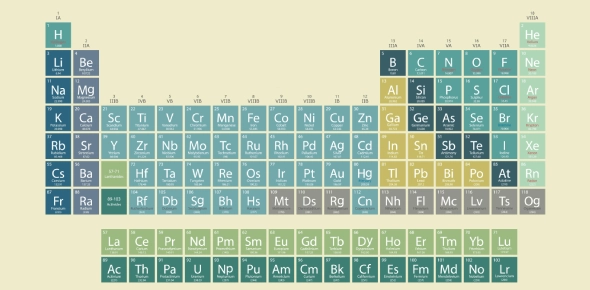
The Periodic Table is a structured chart that organizes all known chemical elements based on their atomic number, electron configuration, and chemical properties. Each element is represented by a box containing its symbol, atomic number, and atomic mass.
Developed by Dmitri Mendeleev in 1869, the table revealed that elements show recurring patterns, or periodicity, in their behavior. Mendeleev arranged elements in order of increasing atomic mass, but today's modern version uses atomic number (number of protons) as the organizing principle.
Key Features:
- Rows are called periods and show increasing atomic numbers.
- Columns are called groups or families and contain elements with similar chemical behaviors.
- The table is divided into metals, nonmetals, and metalloids, as well as blocks based on orbital types (s, p, d, f).
The Periodic Table is an essential tool in chemistry, helping scientists predict how elements will react, bond, and form compounds. It's a visual summary of the building blocks of matter and how they relate to one another.
How Is the Periodic Table Classified?
The periodic table is classified based on patterns in atomic structure, chemical properties, and electron configurations. Its layout helps scientists quickly understand how elements behave and interact.
By Groups (Columns)
The vertical columns are called groups or families. Elements in the same group have similar chemical properties because they have the same number of valence electrons.
- Group 1: Alkali metals (highly reactive, soft metals)
- Group 2: Alkaline earth metals
- Groups 3–12: Transition metals
- Group 17: Halogens (very reactive nonmetals)
- Group 18: Noble gases (inert, stable gases)
By Periods (Rows)
The horizontal rows are called periods. Elements in the same period have the same number of electron shells, but different numbers of electrons in the outer shell. As you move left to right, atomic number increases and properties gradually change.
By Element Types
- Metals: Good conductors, malleable, ductile (found on the left and center)
- Nonmetals: Poor conductors, brittle (found on the right)
- Metalloids: Have properties of both metals and nonmetals (form a "stair-step" line between metals and nonmetals)
By Blocks
The table is also divided into blocks based on the electron sublevel being filled:
- s-block: Groups 1–2 and helium
- p-block: Groups 13–18
- d-block: Transition metals
- f-block: Lanthanides and actinides (bottom two rows)
This classification system allows chemists to predict the reactivity, bonding behavior, and physical properties of elements with accuracy and efficiency.

Fig: The Modern Periodic Table of Elements
Take These Quiz
What Are the Element Symbols and Their Significance?
Element symbols are shorthand notations used to represent chemical elements. Each symbol consists of one or two letters-usually derived from the element's English or Latin name-and is universally recognized in chemistry and science.
Structure of Element Symbols
- The first letter is always capitalized.
- The second letter, if present, is lowercase.
Examples:
- H for Hydrogen
- O for Oxygen
- Na for Sodium (from Natrium, its Latin name)
- Fe for Iron (Ferrum in Latin)
Significance of Element Symbols
- Universal Language: They allow scientists across the world to communicate clearly and consistently, regardless of language.
- Simplifies Notation: Chemical equations and formulas are easier to write and interpret using symbols (e.g., H₂O instead of "two hydrogen atoms and one oxygen atom").
- Identifies Atomic Structure: Each symbol corresponds to a specific atomic number and position in the periodic table, revealing information about the element's protons, electrons, and reactivity.
- Used in Formulas and Reactions: Symbols are used in chemical formulas (CO₂, NaCl), reaction equations, and molecular structures to represent how elements combine and interact.
In essence, element symbols are a compact, standardized way to represent the building blocks of matter in chemistry and science.
What Are the Chemical Properties of Elements in the Periodic Table?
The chemical properties of elements in the periodic table are characteristics that determine how an element behaves during a chemical reaction. These properties are largely influenced by the element's atomic structure, especially the number of valence electrons, and they follow predictable patterns across periods and groups.
Reactivity
- Group 1 (Alkali Metals): Very reactive, especially with water; reactivity increases down the group.
- Group 17 (Halogens): Highly reactive nonmetals; reactivity decreases down the group.
- Group 18 (Noble Gases): Very low reactivity due to full valence electron shells.
Ionization Energy
- The energy required to remove an electron from an atom.
- Increases across a period (left to right) and decreases down a group.
Electronegativity
- A measure of how strongly an atom attracts electrons in a chemical bond.
- Increases across a period, decreases down a group. Fluorine is the most electronegative element.
Electron Affinity
- The amount of energy released when an atom gains an electron.
- Tends to become more negative (i.e., stronger attraction) across a period.
Metallic and Nonmetallic Character
- Metallic character increases down a group and decreases across a period.
- Nonmetallic character is highest on the upper right (excluding noble gases).
Oxidation States
- Many elements can form positive or negative ions depending on their group.
- Group 1: +1
- Group 2: +2
- Group 16: –2
- Group 17: –1
These properties help chemists predict how elements will react, what compounds they'll form, and how they behave in different conditions. The periodic table is designed to make these patterns easy to identify and use.
Take These Quiz
What Are the Branches and Important Discoveries Related to the Periodic Table?
The periodic table is more than just a chart-it's a foundational tool in science that brings together insights from multiple branches of chemistry and physics. Over time, discoveries across these fields have shaped the periodic table into a comprehensive map of all known elements, revealing patterns that help predict their behavior and interactions.
Branches of Science That Support the Periodic Table
- Inorganic Chemistry
This branch focuses on the properties and behavior of elements, especially metals, nonmetals, and their compounds. It uses the periodic table to classify and predict compound formation and reactivity. - Atomic Physics
Atomic physics delves into the internal structure of atoms-protons, neutrons, and electrons. It explains periodic trends based on atomic number, shell structure, and nuclear properties. - Quantum Chemistry
Using principles of quantum mechanics, this field explains how electron configurations define chemical properties and the layout of the periodic table. It's key to understanding why elements group the way they do. - Physical Chemistry
This field links periodic trends (like electronegativity and ionization energy) with thermodynamics and reaction dynamics. It helps explain the energetic behavior of elements during reactions. - Analytical Chemistry
Analytical chemistry relies on the periodic table for identifying elements through techniques like spectroscopy and mass spectrometry. It plays a role in detecting new elements and confirming their properties.
Milestone Discoveries That Shaped the Periodic Table
- Dmitri Mendeleev's Periodic Table (1869)
Mendeleev organized elements by atomic mass and left gaps for elements yet to be discovered. His ability to accurately predict the properties of missing elements like gallium and germanium validated his approach. - Henry Moseley's Atomic Number Concept (1913)
Moseley's discovery that atomic number-not atomic mass-defines an element's identity resolved irregularities in Mendeleev's table. This gave rise to the modern periodic law. - Periodic Law
The modern periodic law states that the chemical and physical properties of elements repeat periodically when elements are arranged by increasing atomic number. This law underpins the entire structure of the periodic table. - Discovery of Noble Gases
The late 19th-century discovery of helium, neon, and others introduced Group 18, a column of inert gases. Their unique stability expanded the understanding of electron configurations and valence shells. - Expansion with Lanthanides and Actinides
As researchers discovered the rare earth elements and heavy radioactive elements, two new series-the lanthanides and actinides-were added below the main table, forming the f-block. - Synthesis of Superheavy Elements
Scientists have created elements with atomic numbers greater than 92 (transuranic elements) in laboratories. These synthetic elements-like oganesson (Og, Z=118)-extended the table and confirmed theoretical predictions about element stability.
Take These Quiz
Conclusion
In this lesson on the Periodic Table, you explored its development from early classifications to the modern arrangement by atomic numbers. You learned how elements are organized into groups and periods, revealing patterns in their properties and behaviors. We examined the significance of element symbols, trends like atomic radius and electronegativity, and the chemical properties that dictate element interactions.
Rate this lesson:
 Back to top
Back to top

(215).jpg)