Heat Transfer Lesson: A Complete Guide for Students
Lesson Overview
Ever wondered how a hot cup of coffee keeps you warm, or why your phone gets hot while charging? The answer lies in the fascinating world of heat transfer. This heat transfer lesson isn't just about stuffy physics – it's about understanding the unseen force that shapes our world. From the sun's warmth to the cooling systems in your refrigerator, heat transfer is everywhere.
Get ready to understand the physics of how heat moves through different materials and environments. This knowledge will help you boost your physics skills and upskill your ability to solve real-world problems in science and engineering. So, buckle up and prepare to be amazed by the power of heat transfer!
What is Heat Transfer?
Heat transfer is the process by which thermal energy moves from one object or substance to another due to a temperature difference. This energy transfer continues until thermal equilibrium is reached, meaning the temperatures of the involved bodies become equal. Heat transfer can occur in solids, liquids, and gases, and it is governed by the laws of thermodynamics. Understanding heat transfer is essential for designing efficient heating and cooling systems, predicting weather patterns, and solving numerous engineering problems.
Scientists Contributions to Heat Transfer
Many scientists have significantly advanced our understanding of heat transfer, forming the basis of modern thermal physics and engineering. This section highlights key figures whose discoveries and theories have shaped the field, explaining how heat moves through materials and environments and leading to technological advancements and practical applications in daily life.
- Joseph Fourier (1768-1830)
- Contribution: Joseph Fourier is best known for Fourier's Law of Heat Conduction, which describes how heat energy transfers through materials. His work laid the foundation for the field of heat conduction.
- Impact: Fourier's mathematical approach to heat transfer is used extensively in engineering and physics to model and solve heat conduction problems.
- James Prescott Joule (1818-1889)
- Contribution: James Joule's experiments on the mechanical equivalent of heat established the principle of energy conservation and the relationship between heat and mechanical work.
- Impact: Joule's work led to the first law of thermodynamics and the concept that in an isolated system energy cannot be created or destroyed, only transformed.
- Ludwig Boltzmann (1844-1906)
- Contribution: Ludwig Boltzmann made significant contributions to statistical mechanics and thermodynamics. The Stefan-Boltzmann Law, which he co-developed, describes the power radiated from a black body in terms of its temperature.
- Impact: Boltzmann's work is fundamental to understanding radiation heat transfer and thermodynamic properties of gases.
- Sir Isaac Newton (1643-1727)
- Contribution: Newton formulated Newton's Law of Cooling, which describes the rate of heat loss of a body.
- Impact: Newton's principles are widely used to understand and calculate convective heat transfer in various applications.
- Thomas Edison (1847-1931)
- Contribution: Although primarily known for his inventions, Thomas Edison's work on electric light bulbs and electric power distribution involved significant applications of heat transfer principles.
- Impact: Edison's innovations in electrical engineering and heat dissipation are critical in the design and operation of electrical devices.
Let's look at the various types of heat transfers in physics.
Types of Heat Transfers in Physics
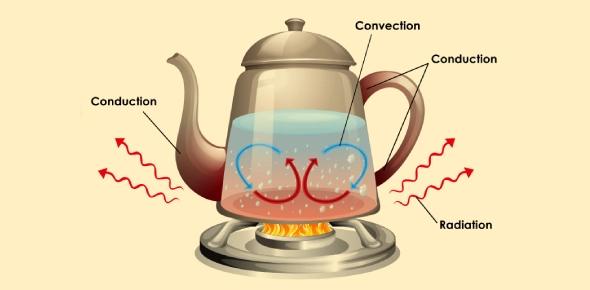
Heat transfer in physics occurs in three primary ways: conduction, convection, and radiation. Each method involves different mechanisms and processes for transferring thermal energy. Let's explore each type in detail:
Conduction
Conduction is the transfer of heat through a solid material due to direct molecular interaction. It occurs when molecules in a substance collide with neighboring molecules, transferring kinetic energy from the hotter region to the cooler region. This transfer of heat continues until thermal equilibrium is reached, meaning the temperature becomes uniform throughout the material.
- Mechanism: In solids, the atoms or molecules are closely packed together, which allows them to transfer heat energy efficiently through vibrations and collisions. This is why conduction is most effective in solids compared to liquids and gases.
- Factors Affecting Conduction:
- Thermal Conductivity: Materials with high thermal conductivity, such as metals (e.g., copper, aluminum), transfer heat more effectively than materials with low thermal conductivity, like wood or plastic.
- Cross-Sectional Area: A larger cross-sectional area allows more heat to flow through the material.
- Temperature Gradient: The greater the temperature difference between the two ends of the material, the faster the heat transfer.
- Thickness: Thinner materials transfer heat more quickly than thicker ones.
- Thermal Conductivity: Materials with high thermal conductivity, such as metals (e.g., copper, aluminum), transfer heat more effectively than materials with low thermal conductivity, like wood or plastic.
- Examples:
- A metal spoon getting hot when placed in a pot of boiling water.
- Heat traveling through the walls of a heated building.
- An iron rod becoming hot at the other end when one end is placed in a flame.
- A metal spoon getting hot when placed in a pot of boiling water.
- Convection
Convection involves the transfer of heat by the movement of fluids, which can be either liquids or gases. It occurs when warmer areas of a fluid rise to cooler areas, creating a circulation pattern that transfers heat throughout the fluid.
- Mechanism: Convection can be natural or forced.
- Natural Convection: This occurs due to the natural buoyancy effects, where warmer, less dense fluid rises, and cooler, denser fluid sinks, creating a convection current. This is seen in boiling water, where hot water rises to the surface, cools, and then sinks.
- Forced Convection: This involves the use of external devices like fans or pumps to create fluid movement and enhance heat transfer. Examples include a ceiling fan circulating air in a room or a pump circulating coolant in a car engine.
- Natural Convection: This occurs due to the natural buoyancy effects, where warmer, less dense fluid rises, and cooler, denser fluid sinks, creating a convection current. This is seen in boiling water, where hot water rises to the surface, cools, and then sinks.
- Factors Affecting Convection:
- Fluid Properties: Viscosity, density, and thermal conductivity of the fluid.
- Temperature Difference: Greater temperature differences enhance convection currents.
- Flow Velocity: Higher flow velocities in forced convection result in increased heat transfer rates.
- Surface Area: Larger surface areas in contact with the fluid enhance heat transfer.
- Fluid Properties: Viscosity, density, and thermal conductivity of the fluid.
- Examples:
- Boiling water in a pot.
- The circulation of warm air in a heated room.
- Ocean currents and atmospheric wind patterns.
- Boiling water in a pot.
- Radiation
Radiation is the transfer of heat through electromagnetic waves without involving particles or a medium. It can occur in a vacuum and does not require a medium for the energy transfer.
- Mechanism: All objects emit and absorb radiant energy in the form of electromagnetic waves. The amount of radiation emitted increases with the temperature of the object. The Stefan-Boltzmann Law quantifies this relationship, stating that the power radiated per unit area is proportional to the fourth power of the object's temperature.
Factors Affecting Radiation:
- The heat from the sun warming the Earth.
- Heat emitted from a fireplace or a radiator.
- Infrared heat lamps used in food warming and reptile enclosures.
Factors Affecting Heat Transfer
Several factors influence the rate and efficiency of heat transfer. Understanding these factors is essential for optimizing thermal management in various applications, from industrial processes to everyday household tasks. Here are the detailed factors that affect heat transfer:
1. Material Properties
The inherent properties of a material play a crucial role in determining how effectively it can transfer heat.
- Thermal Conductivity: This is a measure of a material's ability to conduct heat. Materials with high thermal conductivity, such as metals (e.g., copper, aluminum), can transfer heat rapidly. Conversely, materials with low thermal conductivity, such as wood, plastic, and rubber, are poor conductors of heat and act as insulators. The unit of thermal conductivity is W/m·C (watts per meter per Centigrade).
- Density: The density of a material can influence heat transfer, particularly in convection. In fluids, lower-density fluids tend to rise when heated, while higher-density fluids sink. This creates convection currents that enhance heat transfer. In solids, denser materials may have atoms packed more closely, affecting the efficiency of heat conduction.
- Specific Heat Capacity: This is the amount of heat required to change the temperature of a unit mass of a material by one degree Celsius (or one Kelvin). Materials with high specific heat capacity can absorb and store more heat without a significant change in temperature. For example, water has a high specific heat capacity, making it effective for thermal storage and regulation.
2. Temperature Difference
The rate of heat transfer is directly proportional to the temperature difference between the two bodies or regions involved.
- Greater Temperature Differences: A larger temperature gradient (difference) drives a faster rate of heat transfer. For instance, a hot object placed in a cold environment will lose heat more quickly than in a warmer environment. This principle is governed by Fourier's Law of Heat Conduction, Newton's Law of Cooling, and the Stefan-Boltzmann Law for radiation.
3. Surface Area and Thickness
The physical dimensions of the material or system also significantly affect heat transfer rates.
- Surface Area: A larger surface area provides more space for heat to be transferred. For example, radiators are designed with large surface areas to enhance heat dissipation into the surrounding air. In convection, increasing the surface area in contact with the fluid enhances heat exchange.
- Thickness: The thickness of a material can impede or facilitate heat transfer. In conduction, thinner materials allow heat to pass through more quickly, while thicker materials provide more resistance to heat flow. This relationship is quantified by Fourier's Law, where the heat transfer rate is inversely proportional to the thickness of the material.
4. Medium
The presence and movement of fluids (liquids and gases) can significantly impact the rate of heat transfer, particularly through convection.
- Natural Convection: Involves the movement of fluid due to density differences caused by temperature variations. Warmer, less dense fluid rises, while cooler, denser fluid sinks, creating a convection current. This can be observed in boiling water or atmospheric wind patterns.
- Forced Convection: Involves external forces, such as fans or pumps, to enhance fluid movement and heat transfer. For instance, a fan blowing over a heated surface increases the rate of heat transfer by moving the warm air away and bringing cooler air in contact with the surface.
- Fluid Properties: The viscosity, density, and thermal conductivity of the fluid also affect convection heat transfer. Fluids with lower viscosity and higher thermal conductivity transfer heat more efficiently.
5. Insulation
Insulating materials are used to reduce the rate of heat transfer by providing resistance to conduction, convection, and radiation.
- Insulating Materials: These materials, such as fiberglass, foam, and mineral wool, have low thermal conductivity and are designed to trap air or other gases, which are poor conductors of heat. Insulation is crucial in maintaining desired temperatures within buildings, refrigeration units, and thermal containers.
- Layered Insulation: Multiple layers of insulating material can be used to enhance thermal resistance. The effectiveness of insulation is measured by its R-value, which indicates the material's resistance to heat flow. Higher R-values correspond to better insulating properties.
- Radiative Insulation: Reflective surfaces, such as aluminum foil, can be used to reduce radiative heat transfer by reflecting radiant heat away from the surface.
Applications of Heat Transfer
Heat transfer principles are fundamental to numerous fields and applications, impacting both everyday life and advanced technological systems. Understanding how heat moves and is controlled allows for the design and optimization of various processes and devices. Here are some detailed applications of heat transfer:
1. Engineering
Heat transfer is a critical aspect of many engineering applications. Engineers use the principles of heat transfer to design systems that manage thermal energy efficiently.
- Heat Exchangers: These devices transfer heat between two or more fluids without mixing them. They are used in various industries, including power generation, chemical processing, HVAC (heating, ventilation, and air conditioning), and refrigeration. Examples include shell-and-tube heat exchangers, plate heat exchangers, and air-cooled heat exchangers.
- Engines: Internal combustion engines in cars, airplanes, and industrial machinery rely on heat transfer to convert fuel energy into mechanical work. Efficient heat transfer mechanisms are essential for managing engine temperatures, improving performance, and reducing emissions. Components such as radiators, intercoolers, and exhaust systems are designed to handle and dissipate heat effectively.
- Cooling Systems: Electronic devices, such as computers, smartphones, and data centers, generate significant amounts of heat during operation. Effective cooling systems, including fans, heat sinks, and liquid cooling, are essential to prevent overheating and ensure reliable performance. In power plants, cooling towers and condensers manage the heat generated during electricity production.
2. Meteorology
Heat transfer plays a vital role in understanding and predicting weather patterns and climate changes. The movement of thermal energy in the atmosphere influences various meteorological phenomena.
- Weather Patterns: Convection currents in the atmosphere, driven by the uneven heating of the Earth's surface by the sun, create wind patterns and influence weather systems. Understanding how heat is transferred through the atmosphere helps meteorologists predict storms, hurricanes, and other weather events.
- Climate Change: The study of heat transfer is crucial in climate science to understand the Earth's energy balance and the effects of greenhouse gases. Radiative heat transfer from the sun and the Earth's surface impacts global temperatures and climate patterns. Climate models use heat transfer principles to predict future climate changes and assess the impact of human activities.
3. Environmental Science
Heat transfer is integral to studying natural ecosystems and environmental processes. It affects the distribution of thermal energy in the environment, influencing various ecological and geological phenomena.
- Heat Flow in Ecosystems: In aquatic and terrestrial ecosystems, heat transfer affects the temperature regulation of plants and animals. For example, the temperature of water bodies influences the metabolic rates of aquatic organisms and the overall health of the ecosystem.
- Geothermal Energy: Heat transfer within the Earth's crust is the basis for geothermal energy, a renewable energy source. Geothermal power plants utilize heat from underground reservoirs to generate electricity. Understanding the heat transfer mechanisms in geothermal systems is essential for efficient energy extraction.
- Soil Temperature: The transfer of heat through the soil impacts plant growth and soil microbiology. Soil temperature affects seed germination, root development, and nutrient availability. Agricultural practices often consider soil thermal properties for optimal crop production.
4. Everyday Life
Heat transfer principles are applied in numerous everyday activities and household technologies, enhancing comfort, efficiency, and safety.
- Household Heating and Cooling: HVAC systems use heat transfer principles to regulate indoor temperatures. In heating systems, heat is transferred from a furnace or heat pump to warm the air or water, which is then distributed throughout the home. In cooling systems, air conditioners transfer heat from indoors to outdoors, cooling the indoor environment.
- Cooking: Cooking methods, such as boiling, baking, frying, and grilling, involve heat transfer to prepare food. Understanding how heat transfers through conduction (e.g., frying pan), convection (e.g., oven), and radiation (e.g., grill) helps in achieving desired cooking results.
- Thermal Insulation: Insulation materials in buildings reduce heat loss in winter and heat gain in summer, enhancing energy efficiency and comfort. Insulating walls, roofs, and windows slow down heat transfer, reducing the need for excessive heating or cooling.
- Refrigeration: Refrigerators and freezers use heat transfer principles to keep food and other items cold. By transferring heat from the interior to the exterior environment, these appliances maintain low temperatures inside the compartments.
Formulas Used in Heat Transfer
Understanding the mathematical principles behind heat transfer is essential for analyzing and designing thermal systems. Here are the detailed formulas used in heat transfer, covering conduction, convection, and radiation.
1. Conduction: Fourier's Law of Heat Conduction
Fourier's Law describes the rate of heat transfer through a material by conduction. It is expressed as:
Where
- (-) is the minus sign that tells us that heat flows from large temperatures to lower temperatures.
- Q is the heat transfer rate (Watts, W).
- k is the thermal conductivity of the material (W/m·K), indicating how well the material conducts heat. Higher values mean better conductivity.
- A is the cross-sectional area through which heat is being transferred (m²). A larger area allows more heat to flow.
- dT/dx is the temperature gradient (K/m), representing the rate of temperature change per unit distance. A steeper gradient means a higher rate of heat transfer.
Explanation:
- Thermal Conductivity (k): This property varies for different materials. For instance, metals like copper have high thermal conductivity, while insulating materials like foam have low thermal conductivity.
- Cross-Sectional Area (A): This is the area perpendicular to the direction of heat flow. If you increase this area, more heat can transfer through the material.
- Temperature Gradient (dT/dx): This represents how quickly the temperature changes along the material. A high-temperature difference over a short distance results in a steep gradient, increasing the heat transfer rate.
Example: Imagine a metal rod with one end in contact with a hot source and the other end in contact with a cooler sink. Heat will flow from the hot end to the cool end through conduction. By knowing the thermal conductivity of the rod material, its cross-sectional area, and the temperature difference across its length, you can calculate the rate at which heat is being transferred using Fourier's Law.
2. Convection: Newton's Law of Cooling
Newton's Law of Cooling describes the rate of heat transfer between a solid surface and a fluid (liquid or gas) moving over the surface. It is expressed as:
Q= hA(Ta − Ts)
Where:
- Q is the heat transfer rate (Watts, W).
- h is the convective heat transfer coefficient (W/m²·K), which depends on the fluid properties and flow conditions. Higher values indicate more efficient heat transfer.
- A is the surface area of the object (m²).
- Ts is the surface temperature (K) of the object.
- Tf is the temperature (K) of the fluid.
Explanation:
- Convective Heat Transfer Coefficient (h): This coefficient depends on factors such as the fluid's velocity, viscosity, thermal conductivity, and specific heat capacity. For instance, a high-speed airflow over a surface will have a higher h compared to slow-moving air.
- Surface Area (A): The larger the area of the object in contact with the fluid, the greater the heat transfer. This is why radiators have large surface areas with fins to enhance heat dissipation.
- Temperature Difference (Ta − Ts): The greater the difference between the surface temperature and the fluid temperature, the higher the rate of heat transfer.
Example: Consider a hot cup of coffee left in a cool room. The heat from the coffee will transfer to the surrounding air through convection. By knowing the surface area of the coffee cup, the temperature of the coffee, the room temperature, and the convective heat transfer coefficient, you can calculate how quickly the coffee cools down using Newton's Law of Cooling.
3. Radiation: Stefan-Boltzmann Law
The Stefan-Boltzmann Law describes the power radiated from a black body in terms of its temperature. It applies to the thermal radiation emitted by objects and is expressed as:
Where:
- Q is the heat transfer rate (Watts, W).
- ϵ is the emissivity of the material (dimensionless), which measures how effectively a surface emits radiation compared to a perfect black body. Emissivity values range from 0 to 1.
- σ is the Stefan-Boltzmann constant (5.67×10−8 W/m²·K⁴).
- A is the surface area of the object (m²).
- Ts is the surface temperature (K) of the object.
- Tf is the surrounding temperature (K).
Explanation:
- Emissivity (ϵ): Different materials emit thermal radiation with varying efficiencies. For instance, a polished metal surface has low emissivity, while a matte black surface has high emissivity.
- Surface Area (A): A larger surface area results in more heat being radiated.
- Temperature (Ts ) and (Tf): The heat transfer rate depends on the fourth power of the absolute temperatures of the object and its surroundings. This means that even small temperature differences can result in significant changes in radiative heat transfer.
Example: The Earth receives heat from the sun through radiation. By knowing the surface area of the Earth, its emissivity, and the temperatures involved, scientists can use the Stefan-Boltzmann Law to calculate the amount of heat radiated back into space and understand the Earth's energy balance.
Key Takeaway
Congratulations! You've successfully navigated the cool world of heat transfer. No longer a mystery, the principles of conduction, convection, and radiation are now part of your scientific toolkit. You can confidently explore how heat moves through different materials, analyze various environments, and appreciate the foundational work of pioneering scientists in this field.
The beauty of heat transfer lies in its real-world applications, from understanding why your phone heats up to designing more efficient cooling systems. This course has given you a solid foundation for further exploration and problem-solving. Keep your curiosity alive, ask questions, and apply your newfound knowledge to tackle new thermal challenges.
Rate this lesson:
 Back to top
Back to top


(161).jpg)