Ionic Compounds Formation, Characteristics, & Examples: A Complete Guide with Real-World Relevance
Lesson Overview
Ionic compounds are chemical compounds formed through ionic bonds between positively charged ions (cations) and negatively charged ions (anions). These compounds have distinct properties due to the strong electrostatic forces between oppositely charged ions, which hold them together in a crystal lattice structure. The arrangement of these ions in a regular, repeating pattern is what gives ionic compounds their characteristic physical properties such as hardness, high melting and boiling points, and brittleness.
Ionic bonds are formed when electrons are transferred from one atom to another. This typically occurs between metals, which tend to lose electrons, and non-metals, which gain electrons. The resulting electrostatic attraction between the oppositely charged ions forms a stable ionic compound.
How Are Ionic Compounds Formed?
The formation of ionic compounds involves the transfer of electrons between atoms. Metals, located on the left side of the periodic table, have one or more electrons in their outer shell that are easily lost due to their low ionization energy. Non-metals, on the right side of the periodic table, have higher electronegativity and a stronger tendency to attract electrons.
- Electron Transfer:
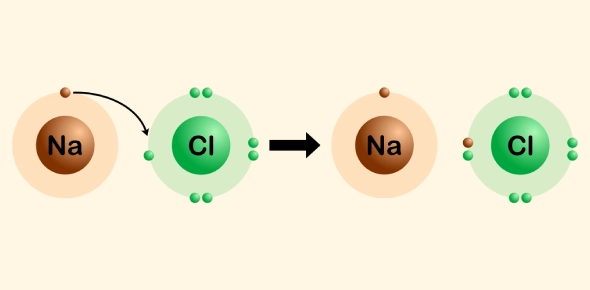
The process begins when a metal atom loses electrons, becoming a positively charged ion (cation). For example, sodium (Na) loses an electron to form a Na⁺ ion. Meanwhile, a non-metal atom gains the electrons, becoming a negatively charged ion (anion). For example, chlorine (Cl) gains an electron to form a Cl⁻ ion.
- Formation of Ionic Bonds:
Once the electron transfer occurs, the oppositely charged ions attract each other through electrostatic forces, forming an ionic bond. The ionic bond is strong, and this attraction stabilizes the ions, creating an ionic compound. Example: In the formation of sodium chloride (NaCl), sodium (Na) loses one electron to form a Na⁺ ion, and chlorine (Cl) gains that electron to form a Cl⁻ ion. The Na⁺ and Cl⁻ ions are then attracted to each other, forming a stable ionic bond and creating the compound NaCl. These ions arrange themselves in a cubic crystal lattice, maximizing attraction and minimizing repulsion.
Stability and Properties of the Formed Ionic Compounds
The stability of ionic compounds is due to the strong electrostatic forces between cations and anions in the crystal lattice. This leads to several key physical properties:
Solubility in Polar Solvents:
Ionic compounds are typically soluble in polar solvents like water because the polar molecules can interact with the charged ions, helping to separate and dissolve them.
High Melting and Boiling Points:
Ionic compounds have high melting and boiling points because the strong ionic bonds require a significant amount of energy to break. This is due to the stable, tightly held ionic bonds in the crystal lattice.
Brittleness:
Although ionic compounds are hard, they are also brittle. Any displacement of ions in the lattice can cause like-charged ions to repel each other, leading to fractures in the structure.
Electrical Conductivity:
Ionic compounds do not conduct electricity in their solid form because the ions are fixed in place within the lattice. However, when ionic compounds are melted or dissolved in water, the ions are free to move and carry charge, allowing them to conduct electricity.
Examples of Ionic Compounds and Their Formation
Calcium Fluoride (CaF₂)
In calcium fluoride, calcium (Ca) loses two electrons to form Ca²⁺ ions, while fluorine (F) gains one electron to form F⁻ ions. Two F⁻ ions pair with each Ca²⁺ ion, resulting in the ionic compound CaF₂. This structure is highly stable and strong.
Sodium Chloride (NaCl)
Sodium chloride is formed when sodium (Na) transfers one electron to chlorine (Cl). The Na⁺ and Cl⁻ ions are attracted to each other, forming the stable ionic compound NaCl. The ions arrange in a cubic lattice structure.
Magnesium Oxide (MgO)
Magnesium (Mg) has two electrons in its outer shell, which it loses to become Mg²⁺. Oxygen (O) has six electrons in its outer shell and gains two electrons to become O²⁻. The resulting Mg²⁺ and O²⁻ ions attract each other, forming magnesium oxide (MgO), a compound with high melting and boiling points.
Take This Quiz
What Is the Difference Between Ionic and Covalent Compounds?
Ionic and covalent compounds are two major types of chemical compounds, differing in how their atoms bond and the resulting properties.
- Bond Formation:
- Ionic Compounds: Formed when electrons are transferred from one atom (typically a metal) to another atom (typically a non-metal), creating positively and negatively charged ions that attract each other.
- Example: In NaCl, sodium (Na) loses an electron to chlorine (Cl), forming Na⁺ and Cl⁻, which are held together by electrostatic forces.
- Example: In NaCl, sodium (Na) loses an electron to chlorine (Cl), forming Na⁺ and Cl⁻, which are held together by electrostatic forces.
- Covalent Compounds: Formed when atoms share electrons to achieve stable electron configurations, usually between non-metal atoms.
- Example: In H₂O, oxygen shares electrons with hydrogen to form covalent bonds.
- Example: In H₂O, oxygen shares electrons with hydrogen to form covalent bonds.
- Ionic Compounds: Formed when electrons are transferred from one atom (typically a metal) to another atom (typically a non-metal), creating positively and negatively charged ions that attract each other.
- Structure and Composition:
- Ionic Compounds: Have a crystalline lattice structure, with ions arranged in a regular, repeating pattern.
- Example: NaCl forms a cubic lattice, where each Na⁺ ion is surrounded by six Cl⁻ ions.
- Example: NaCl forms a cubic lattice, where each Na⁺ ion is surrounded by six Cl⁻ ions.
- Covalent Compounds: Typically exist as individual molecules with distinct boundaries, though some form networks.
- Example: CO₂ exists as discrete molecules, each formed by two oxygen atoms double-bonded to one carbon atom.
- Example: CO₂ exists as discrete molecules, each formed by two oxygen atoms double-bonded to one carbon atom.
- Ionic Compounds: Have a crystalline lattice structure, with ions arranged in a regular, repeating pattern.
- Physical Properties:
- Ionic Compounds: Tend to have high melting and boiling points, are often soluble in water, and can conduct electricity when molten or dissolved.
- Example: Sodium chloride has a melting point of 801°C and conducts electricity when dissolved in water.
- Example: Sodium chloride has a melting point of 801°C and conducts electricity when dissolved in water.
- Covalent Compounds: Generally have lower melting and boiling points, are less soluble in water, and do not conduct electricity.
- Example: Methane (CH₄) is a gas at room temperature with a boiling point of -161.5°C.
- Example: Methane (CH₄) is a gas at room temperature with a boiling point of -161.5°C.
- Ionic Compounds: Tend to have high melting and boiling points, are often soluble in water, and can conduct electricity when molten or dissolved.
- Electrical Conductivity:
- Ionic Compounds: Conduct electricity in their molten or dissolved state due to the mobility of ions.
- Covalent Compounds: Do not conduct electricity as they lack free ions or electrons.
- Ionic Compounds: Conduct electricity in their molten or dissolved state due to the mobility of ions.
What Are the Properties of Ionic Compounds?
Ionic compounds have several distinctive properties:
- High Melting and Boiling Points:
Due to the strong electrostatic forces holding the ions together, ionic compounds generally require a high amount of energy to melt or boil. - Electrical Conductivity:
Ionic compounds can conduct electricity when they are dissolved in water or melted, as the ions are free to move and carry charge. - Solubility:
Ionic compounds are usually soluble in water but insoluble in non-polar solvents. - Hardness and Brittleness:
Ionic compounds are hard due to the strong ionic bonds, but they are brittle. Any slight shift in the lattice can cause fractures. - Crystalline Structure:
Ionic compounds form crystal lattices where ions of opposite charges are arranged in a highly ordered, repeating pattern.
What Are the Applications of Ionic Compounds?
Ionic compounds are widely used in various industries due to their unique properties:
- Medical and Pharmaceutical Applications:
- Electrolyte Solutions: Ionic compounds like potassium chloride (KCl) are used in IV fluids to treat or prevent potassium deficiency.
- Antacids: Magnesium hydroxide (Mg(OH)₂) is used as an antacid to neutralize stomach acid.
- Imaging: Barium sulfate (BaSO₄) is used in X-ray imaging of the gastrointestinal tract.
- Electrolyte Solutions: Ionic compounds like potassium chloride (KCl) are used in IV fluids to treat or prevent potassium deficiency.
- Industrial Applications:
- Metallurgy: Calcium chloride (CaCl₂) is used in metal extraction and as a de-icing agent.
- Glass Manufacturing: Sodium carbonate (Na₂CO₃) lowers the melting point of silica in glassmaking.
- Ceramics: Magnesium oxide (MgO) is used in refractory materials for high-temperature processes.
- Metallurgy: Calcium chloride (CaCl₂) is used in metal extraction and as a de-icing agent.
- Agricultural Applications:
- Fertilizers: Ionic compounds like ammonium nitrate (NH₄NO₃) provide essential nutrients such as nitrogen for plant growth.
- Soil Conditioning: Calcium sulfate (CaSO₄) improves soil structure and reduces compaction.
- Fertilizers: Ionic compounds like ammonium nitrate (NH₄NO₃) provide essential nutrients such as nitrogen for plant growth.
- Water Treatment:
- Water Softening: Sodium chloride (NaCl) and sodium carbonate (Na₂CO₃) are used to remove calcium and magnesium ions, making water "soft."
- Disinfection: Calcium hypochlorite (Ca(ClO)₂) is used in water sterilization and pool cleaning.
- Water Softening: Sodium chloride (NaCl) and sodium carbonate (Na₂CO₃) are used to remove calcium and magnesium ions, making water "soft."
- Food Industry:
- Preservation: Sodium nitrate (NaNO₃) is used as a preservative in cured meats.
- Leavening Agents: Sodium bicarbonate (NaHCO₃) is used in baking to release carbon dioxide for dough rising.
- Preservation: Sodium nitrate (NaNO₃) is used as a preservative in cured meats.
Take This Quiz
Rate this lesson:
 Back to top
Back to top
(477).jpg)